What is an atomic mass of NaOH ?
1 Answer
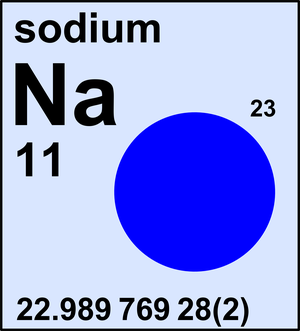
. Note that the molar mass of carbon-12 (in grams) is numerically equal to its atomic mass in amu. Likewise, the atomic mass of sodium (Na) is 22.99 amu and its molar mass is 22.99 g; the atomic mass of phosphorus is 30.97 amu and its molar mass is 30.97 g; and so on. If we know the atomic mass of an element, we also know its molar mass. Atomic Mass of Sodium Atomic mass of Sodium is 22.9897 u. Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance. The unit of measure for mass is the atomic mass unit (amu). Sodium has been identified in both the atomic and ionic forms in the spectra of stars, including the Sun, and the interstellar medium. Analysis of meteorites indicates that the silicate material present has an average content of approximately 4.6 atoms of sodium for every 100 atoms of silicon.
Well, what is the molar mass of sodium hydroxide....?
Atomic Number of Sodium. Atomic Number of Sodium is 11. Chemical symbol for Sodium is Na. Number of protons in Sodium is 11. Atomic weight of Sodium is 8 u or g/mol. Melting point of Sodium is 97,8 °C and its the boiling point is 892 °C. Thus, since the atomic mass of iron is 55.847 amu, one mole of iron atoms would weigh 55.847 grams. The same concept can be extended to ionic compounds and molecules. One formula unit of sodium chloride (NaCl) would weigh 58.44 amu (22.98977 amu for Na + 35.453 amu for Cl), so a mole of sodium chloride would weigh 58.44 grams.

Explanation:
The molar mass of sodium hydroxide is
But this, BY DEFINITION, specifies the mass of

And thus the mass of ONE formula unit of sodium hydroxide is given by the quotient....
Atomic Mass Of Sodium And Potassium
Related questions
